Health
PEPFAR and Gilead Collaborate to Expand Access to Lenacapavir for HIV Prevention
The President’s Emergency Plan for AIDS Relief (PEPFAR) and Gilead Sciences revealed plans to purchase injectable lenacapavir for HIV prevention (PrEP) in countries with the highest HIV/AIDS burdens, focusing on preventing transmission from mothers to their children.
Mitchell Warren, the executive director of the AIDS Vaccine Advocacy Coalition (AVAC), a global HIV prevention organization, remarked, “Prevention programs were absolutely gutted.” He emphasized that the lenacapavir announcement signals an important step, indicating that PEPFAR and the U.S. government are once again prioritizing PrEP.
Uganda, a country with high HIV/AIDS rates, has benefited from PEPFAR’s initiatives in the past, including significant successes in preventing mother-to-child transmission of HIV. Though the U.S. Agency for International Development (USAID) is no longer active in Uganda, the continued support from PEPFAR remains crucial.
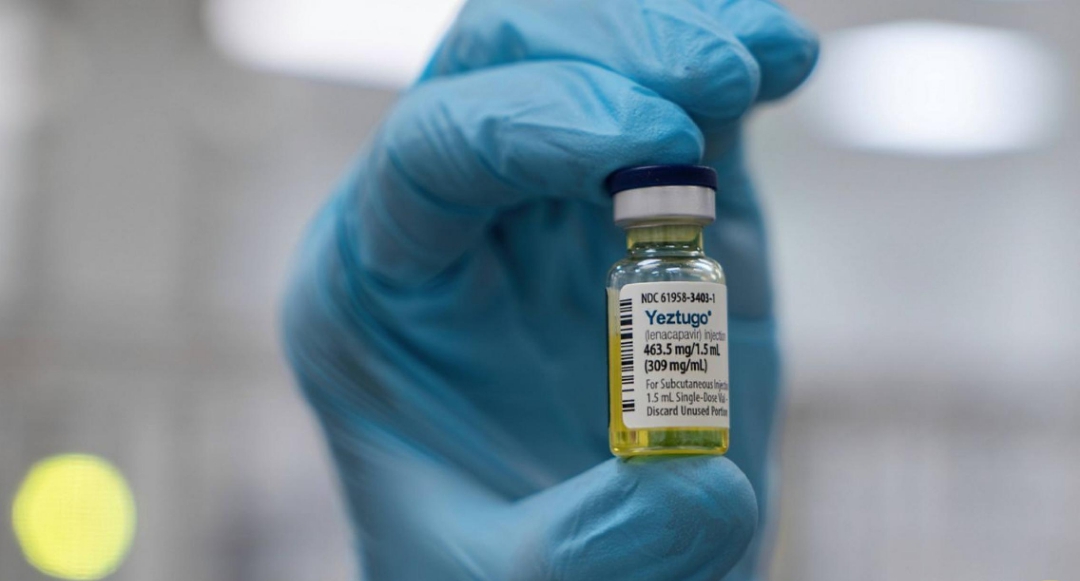
Daniel O’Day, Chairman and CEO of Gilead Sciences, emphasized the potential impact of this partnership, stating, “The U.S. State Department’s support through PEPFAR will accelerate access to lenacapavir and move us closer to ending the HIV epidemic.” He also highlighted lenacapavir as a groundbreaking scientific achievement, developed by Gilead scientists over nearly two decades.
In clinical trials, lenacapavir proved to be highly effective, with over 99% of participants remaining HIV-negative. The drug shows particular promise for pregnant and breastfeeding women, as it offers safe protection during and after pregnancy, thus preventing mother-to-child transmission.
Clinical trial data from last year indicated that twice-yearly injections of lenacapavir could offer near-complete protection against HIV for individuals at risk, including for those aiming to prevent mother-to-child transmission.
Despite this significant announcement, AVAC’s Mitchell Warren pointed out that while PEPFAR’s renewed involvement is encouraging, many critical issues remain.
Though Uganda was involved in clinical trials for the breakthrough injectable HIV prevention drug, concerns persist about its accessibility due to its high cost.
Winnie Byanyima, Executive Director of UNAIDS, along with medicine and vaccines advocates like AVAC, has called on the U.S. and Gilead to reduce the cost of lenacapavir to ensure broader access. The U.S. has long been a global leader in the fight against HIV/AIDS.
Since its creation in 2003, PEPFAR has received bipartisan support and has invested more than $120 billion to combat HIV/AIDS, making it the largest commitment any country has ever made to fight a single disease.
Comments