*Some of the dangerous drugs block blood streams of patients
*Others cannot prevent pain during major surgical operations
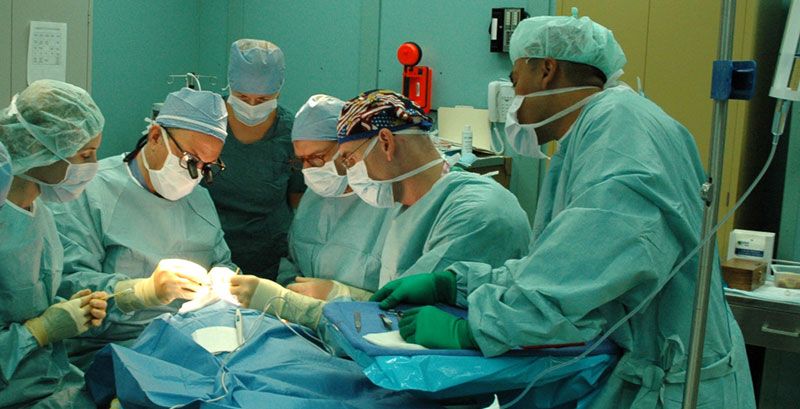
If you’ve heard of a friend, wife or relative who was forced to endure excruciating pain during an operation, > he/she was likely a victim of fake medicines that was until recently, being sold by pharmacies in Uganda.
The National Drug Authority (NDA) this week made shocking revelations that drug importers had violated minimum quality standards by importing sub-standard drugs that put the lives of Ugandans in serious danger.
NDA officials told a news conference that six companies have been involved in the importation of fake drugs that did not meet their minimum safety standards.
NDA outlined a list of six drugs which it banned; they are Univittal (a multivitamin), Hydrapres injection, Agonal, Vitamin B Complex Lyopox+PPR, an animal vaccine as well as Netvaicain.
Dr. Peter Ssali, the Head of Quality at the National Drug Authority revealed that the authority has recalled and indefinitely banned the six drugs following complaints from patients and clinicians.
Ssali explained that some of the drugs were found to be containing unusual substances and others labeled with wrong prescriptions
NDA has also threatened to withdraw dealership licenses from companies whose identities were not disclosed.
“Protecting the human and animal population from unsafe drugs is a collective responsibility, we advise the public to be vigilant and look out for fake drugs, Ssali shockingly revealed that the wrongly labelled Bupivacaine Injection meant to be used as anesthesia during an operation but fails to stop the pain.
Ssali assured clinicians and Pharmacy owners who might have purchased those drugs that they will not make losses if they produce the available drugs in stock and details of the amount they purchased. He said that they are going to make suppliers to refund their money.
The NDA recalled selected veterinary drugs from the market after discovering they cause cancer to humans. They included Cospro-F, a water soluble powder for chicken, Fuzol, Neoceryl and Appealyte which wasn’t registered for sale on the Ugandan market.
On 18 July 2016, National Drug Authority (NDA) warned against of Counterfeit Malarial drug called Coartem.














Sunrise reporter
Leave a Comment
Your email address will not be published.